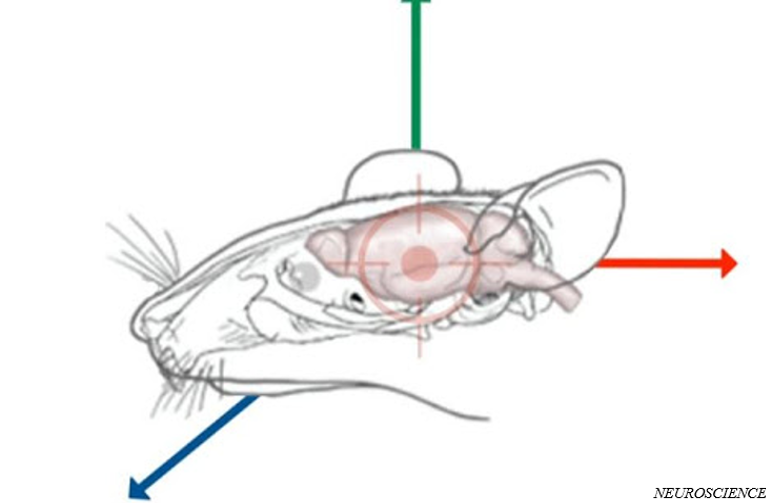
Stereotactic injection is a useful technique to deliver high titer viruses to targeted brain areas in mice. Take the injection into the rostral anterior cingulate cortex (rACC) of mice as the example, the experimental process of intracerebral viral injection is as follows.
Prepping mice for surgery
● Before surgery, mouse is anesthetized by intraperitoneal injection of 2 % sodium pentobarbital (50 mg/kg). Wait until the mouse becomes motionless (~5 min).
● Remove the fur on the mouse’s head where the incision is planned, using electric shaver for small animals.
● Mounting it to the stereotaxic apparatus, then sterilize the skin surface on the head with iodophor and 75 % ethanol in turn.
● Make 0.8-cm-long incision on the mouse’s head and push the transparent mucosal membrane covering the skull to both sides of the incision, thereby exposing part of the surface of the skull.
Target coordinates
● Clean the skull with saline and dry it with ear syringe, exposing the location of Bregma and Lambda. Taking the Bregma as the reference zero point and adjusting the skull plane, ensure that the left and right sides of the skull as well as the Bregma and Lambda are at the same height.
● Determine and mark the rACC injection site according to brain atlas of the mouse, A/P= +1.6 mm, M/L=+/- 0.3 mm and D/V= -1.7 mm.
Equipment setup
● Start drilling a small hole according to the marked positions in the skull.
● Uniform injection is performed by using the micro-injection pump controlled by the micro-injection controller (which is mounted to the stereotactic apparatus), allowing the syringe pump to be vertical to the brain.
● Draw glass capillary tubing for syringe pump into an electrode tube with a suitable tip length and thickness through the vertical electrode puller. Then fill the electrode tube with mineral oil or double distilled water (ddH
2O) and fix it on the syringe pump.
● Before injection, use the micro syringe to aspirate 0.5 μl of air, 0.5 μl of NS, and 0.5 μl of air in turn, test whether the micro syringe is unobstructed in the air.
● Lower the electrode tip perpendicular to the desired depth based on the brain atlas coordinates, and start the injection with the micro-injection pump at a rate of 0.05 μl/min.
Post-injection care
● After the injection is finished, wait for about 10 min to ensure that any residual virus has been absorbed. Slowly raise the syringe and use iodophor to sterilize the injection site and its vicinity, then place it next to the electric heater until it recovers.
● Allow more than 2 weeks for the virus to fully express in the brain before arranging the next related experiment. In general, behavioral test is recommended to be carried out within 4 weeks of virus expression.
NOTE:
AP: Aterior-posterior,“+”means aterior,“-”means posterior
ML: Medial-lateral,“+”means medial,“-”means lateral
DV: Dorsal-ventral,“+”means dorsal,“-”means ventral
The methods of virus injection in various site are similar, just alter the injection dosage according to the characteristics and titer of different viruses. It is recommended to conduct pre-experiment before using each virus.
If you have any questions,please email us at
[email protected].