Plaque assays remain one of the most accurate methods for the direct quantification of infectious virons and antiviral substances through the counting of discrete plaques in cell culture. During a plaque assay, a confluent monolayer of host cells is infected with a lytic virus of an unknown concentration which has been serially diluted to a countable range, typically between 5-100 virions. Then, infected monolayers are covered with solid or semisolid overlay substrates, such as agarose or carboxymethyl cellulose, have been used to restrict viral spread, preventing indiscriminate infection through the liquid growth medium.
After the initial infection and application of the immobilizing overlay, individual plaques, or zones of cell death, will start to develop as viral infection and replication are constrained to the surrounding monolayer. Infected cells will continue the replication-lysis-infection cycle, further propagating the infection, leading to increasingly distinct and discrete plaques. According to the viral growth kinetics and host cell used, a visible plaque will ordinarily form within 2-14 days. After fixing and staining the infected cellular monolayer, plaques are counted so that titer viral stock samples in terms of plaque forming units (pfu) per milliliter.
Protocol:
1. The day prior to the assay, plate appropriate host cells in wells. The next day, Allow the cells to reach at 90 - 100% confluency.
2. Check the confluency and viability of the cells prior to starting the assay. Ensure standard cellular morphology and a ~90% confluent monolayer is present .
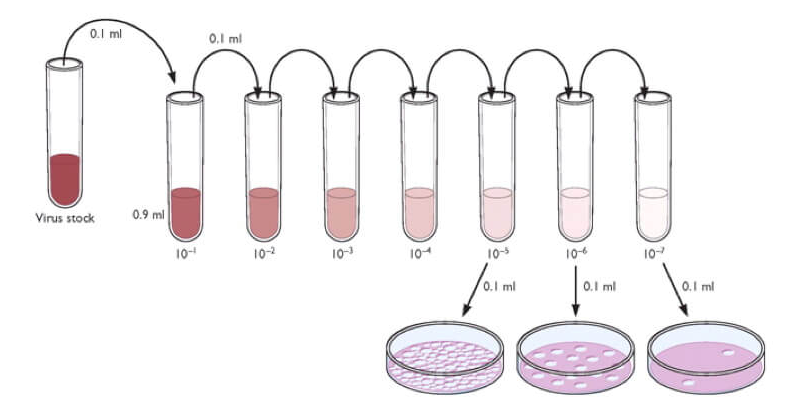
3. Perform a tenfold serial dilution of the infectious samples. Use the cellular growth media for viral propagation as the diluent. Vary the number of dilutions required based on the expected titer of the virus in question, and always use an uninfected control sample to independently ensure cellular viability and aid in plaque identification.
4. Remove the growth medium and wash with Phosphate-Buffered Saline (PBS). Add diluted virus to each well, using multiple wells per dilution.
5. Incubate dishes to make viral adsorption. Remove the inoculum and wash with basal medium.
6. Overlay the cells with overlay medium. Incubate for a length of time appropriate for infection. If applicable, remove overlay.
7. Observe the cell monolayers every day for the presence of foci or plaques.
8. In order to determine the virus titer, the plaques are counted. A log drop should be noted between serial dilutions and, depending on plate size, between 5-100 plaques counted, with a negative control used as a reference.
9. Determine the viral titer of the stock sample by taking the average number of plaques for a dilution and the inverse of the total dilution factor. Then use the following formula to determine the titer (pfu/ml) of the viral stock:
pfu/ml = Avg. of Plaques / (D x V)
D = Dilution factor V = Volume of diluted virus/well
Sample calculation:
- An average of 100 plaques formed in the 1:20,000 dilution wells.
- Volume of diluted virus added: 0.1ml
= 100/ (0.0002 x 0.1) = 5 x 10
6 pfu/ml