AAV2/retro was used to retrograde targeting the CSN innervating the lumbar spinal cord. (From
BrainVTA)
The viruses used in this article from BrainVTA are in the table below
|
Control |
PT-0105 AAV-CAG-mCherry |
Yang Yang, Xuezhu Chen, Zhizhong Feng, Xianfeng Cai, Xiaoming Zhu, Ming Cao, Likun Yang, Yujie Chen, Yuhai Wang, Hua Feng
Pub Date: 2021-08-18,
DOI: 10.1111/jnc.15493,
Email: [email protected]
Injury to long axonal projections is a central pathological feature at the early phase of intracerebral hemorrhage (ICH). It has been reported to contribute to persistent functional disability following ICH. However, the molecular mechanisms that drive axonal degeneration remain unclear. Autologous blood was injected into the striatum to mimic the pathology of ICH. Observed significant swollen axons with characteristic retraction bulbs were found around the striatal hematoma at 24 h after ICH. Electronic microscopic examination revealed highly disorganized microtubule and swollen mitochondria in the retraction bulbs. MEC17 is a specific α-tubulin acetyltransferase, ablation of acetylated α-tubulin in MEC17−/− mice aggravated axonal injury, axonal transport mitochondria dysfunction, and motor dysfunction. In contrast, treatment with tubastatin A (TubA), which promotes microtubule acetylation, significantly alleviated axonal injury and protected the integrity of the corticospinal tract and fine motor function after ICH. Moreover, results showed that 41% mitochondria were preferentially bundled to the acetylated α-tubulin in identifiable axons and dendrites in primary neurons. This impaired axonal transport of mitochondria in primary neurons of MEC17−/− mice. Given that opening of mitochondrial permeability transition pore (mPTP) induces mitochondrial dysfunction and impairs ATP supply thereby promoting axonal injury, we enhanced the availability of acetylated α-tubulin using TubA and inhibited mPTP opening with cyclosporin A. The results indicated that this combined treatment synergistically protected corticospinal tract integrity and promoted fine motor control recovery. These findings reveal key intracellular mechanisms that drive axonal degeneration after ICH and highlight the need to target multiple factors and respective regulatory mechanisms as an effective approach to prevent axonal degeneration and motor dysfunction after ICH.
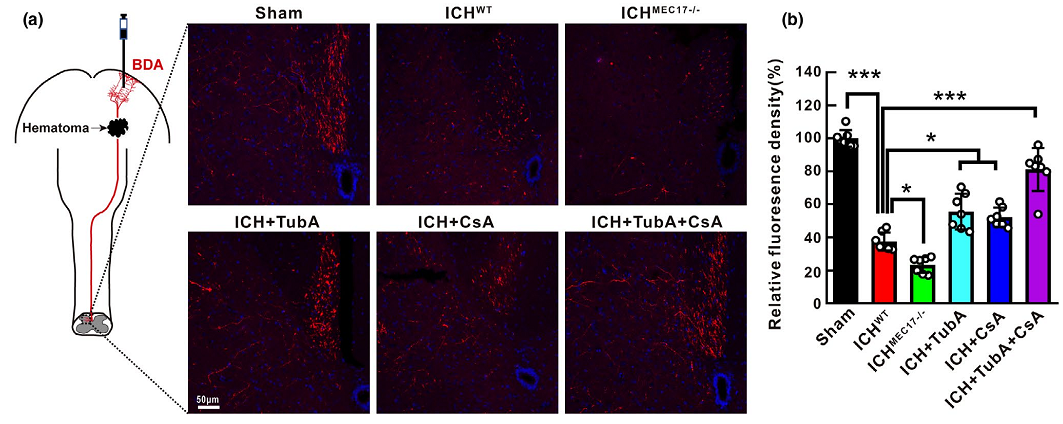 Figure 1. Schematic diagram and representative immunofluorescence staining pictures of anterogradely BDA traced corticospinal tract in the spinal cord of each mice after ICH.
Figure 1. Schematic diagram and representative immunofluorescence staining pictures of anterogradely BDA traced corticospinal tract in the spinal cord of each mice after ICH.
In this study, the authors explored the pathomorphological characteristics of axonal injury following ICH at the acute stage and the protective function of α- Ac-Tub in early axonal degeneration. In addition, they aimed to identify novel therapeutic targets for inhibiting microtubule disassembly and axonal injury to devise more promising therapeutic strategies with combinatorial manipulation of multiple factors to promote functional recovery of injured axons.
BrainVTA offers viral vector construction & virus packaging services for AAV, LV, RABV, PRV, HSV and VSV that help researchers explore questions about genes, neurons, circuitry structure, function of brain network, mechanism and treatment of diseases.
If you have any needs, just email us at
[email protected].
