siRNA-mediated TFEB was used for gene knockdown. (From
BrainVTA)
The viruses used in this article from BrainVTA are in the table below
|
Custom-Made services |
TFEB-siRNA
siRNA NC |
Ling Zhou, Zeming Liu, Sichao Chen, Jing Qiu, Qianqian Li, Shipei Wang, Wei Zhou, Danyang Chen, Guang Yang, Liang Guo
Pub Date: 2020-12-09,
DOI: 10.3892/ijmm.2020.4814,
Email: [email protected]
Autophagy is reported to be involved in the formation of skin hypertrophic scar (HTS). However, the role of autophagy in the process of fibrosis remains unclear, therefore an improved understanding of the molecular mechanisms associated with autophagy may accelerate the development of effective therapeutic strategies against HTS. The present study evaluated the roles of autophagy mediated by transcription factor EB (TFEB), a pivotal regulator of lysosome biogenesis and autophagy, in transforming growth factor‑β1 (TGF‑β1)‑induced fibroblast differentiation and collagen production. Fibroblasts were treated with TGF‑β1, TGF‑β1 + tauroursodeoxycholic acid (TUDCA) or TGF‑β1 + TFEB‑small interfering RNA (siRNA). TGF‑β1 induced phenotypic transformation of fibroblasts, as well as collagen synthesis and secretion in fibroblasts in a dose‑dependent manner. Western blotting and immunofluorescence analyses demonstrated that TGF‑β1 upregulated the expression of autophagy‑related proteins through the endoplasmic reticulum (ER) stress pathway, whereas TUDCA reversed TGF‑β1‑induced changes. Reverse transcription‑quantitative PCR (RT‑qPCR), western blotting and RFP‑GFP‑LC3 double fluorescence analyses demonstrated that knockdown of TFEB by TFEB‑siRNA decreased autophagic flux, upregulated the expression of proteins involved in the apoptotic pathway, such as phosphorylated‑α subunit of eukaryotic initiation factor 2, C/EBP homologous protein and cysteinyl aspartate specific proteinase 3, and also downregulated the expression of α‑smooth muscle actin and collagen I (COL I) in fibroblasts. Immunofluorescence confocal analyses and enzyme‑linked immunosorbent assay indicated that TGF‑β1 increased the colocalization of COL I with lysosomal‑associated membrane protein 1 and Ras‑related protein Rab‑8A, a marker of secretory vesicles, in fibroblasts, as well as the secretion of pro‑COL Iα1 in culture supernatants. Meanwhile, these effects were abolished by TFEB knockdown. The present results suggested that autophagy reduced ER stress, decreased cell apoptosis and maintained fibroblast activation not only through degradation of misfolded or unfolded proteins, but also through promotion of COL I release from the autolysosome to the extracellular environment.
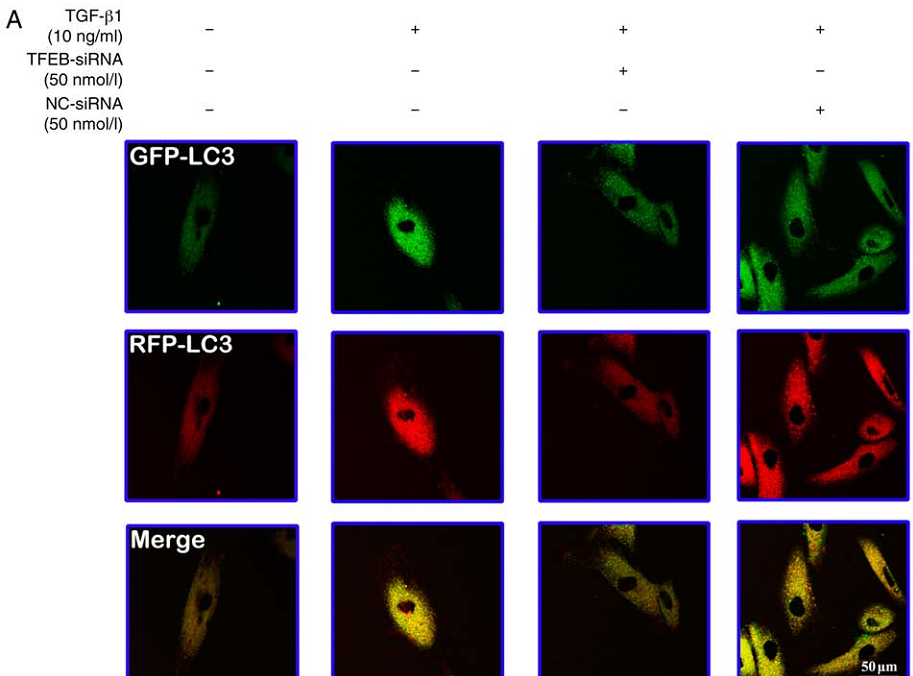 Figure 1. TFEB-siRNA inhibits TGF-β1‑induced enhancement of autophagic flux.
Figure 1. TFEB-siRNA inhibits TGF-β1‑induced enhancement of autophagic flux.
In the present study, using TGF-β1‑treated human skin fibroblasts as a model system, the aim was to investigate whether TFEB mediated-autophagy was involved in fibroblast differentiation and collagen production, and to explore the underlying molecular mechanism in this process, and thus provide a novel perspective for the development of strategies for the treatment of HTS.
BrainVTA offers viral vector construction & virus packaging services for AAV, LV, RABV, PRV, HSV and VSV that help researchers explore questions about genes, neurons, circuitry structure, function of brain network, mechanism and treatment of diseases.
If you have any needs, just email us at
[email protected].
