Cre-dependent monosynaptic retrograde tracing system was used to check and detail the VTA
VGluT2+ network. (From
BrainVTA)
The viruses used in this article from BrainVTA are in the table below
|
AAV tracing helper |
AAV2/9-CAG-DIO-histone-TVA-GFP
PT-0519 AAV2/9-CAG-DIO-RV-G |
|
RV |
EnvA-RV-DsRed |
Quentin Montardy, Zheng Zhou, Zhuogui Lei, Xuemei Liu, Pengyu Zeng, Chen Chen, Yuanming Liu, Paula Sanz-Leon, Kang Huang, Liping Wang
Pub Date: 2019-05-13,
DOI: 10.1016/j.scib.2019.05.005,
Email: [email protected]
The Ventral Tegmental Area (VTA) is a midbrain structure known to integrate aversive and rewarding stimuli, but little is known about the role of VTA glutamatergic (VGluT2) neurons in these functions. Direct activation of VGluT2 soma evokes rewarding behaviors, while activation of their downstream projections evokes aversive behaviors. To facilitate our understanding of these conflicting properties, we recorded calcium signals from VTAVGluT2+ neurons using fiber photometry in VGluT2-cre mice to investigate how this population was recruited by aversive and rewarding stimulation, both during unconditioned and conditioned protocols. Our results revealed that, as a population, VTAVGluT2+ neurons responded similarly to unconditioned-aversive and unconditioned-rewarding stimulation. During aversive and rewarding conditioning, the CS-evoked responses gradually increased across trials whilst the US-evoked response remained stable. Retrieval 24 h after conditioning, during which mice received only CS presentation, resulted in VTAVGluT2+ neurons strongly responding to CS presentation and to the expected-US but only for aversive conditioning. To help understand these differences based on VTAVGluT2+ neuronal networks, the inputs and outputs of VTAVGluT2+ neurons were investigated using Cholera Toxin B (CTB) and rabies virus. Based on our results, we propose that the divergent VTAVGluT2+ neuronal responses to aversion and reward conditioning may be partly due to the existence of VTAVGluT2+ subpopulations that are characterized by their connectivity.
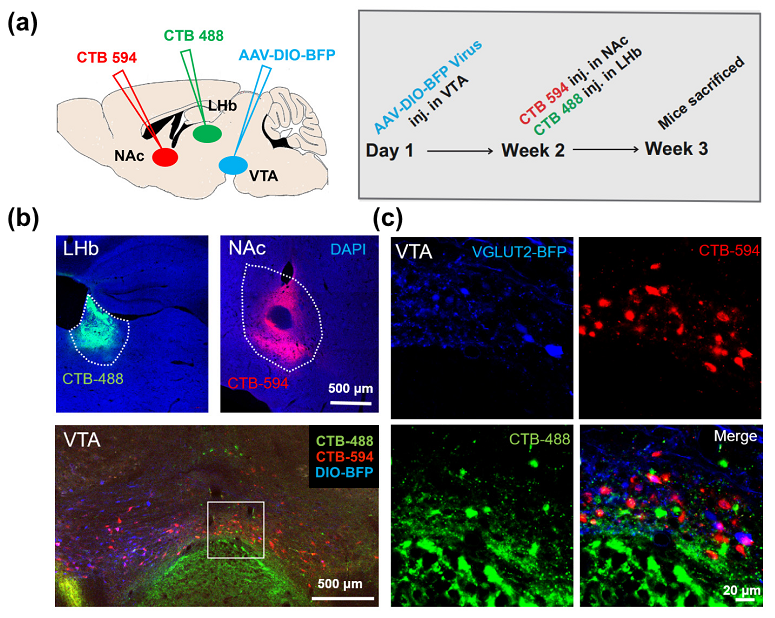 Figure 1. LHb and NAc projections to VTA VGluT2 neurons.
Figure 1. LHb and NAc projections to VTA VGluT2 neurons.
In this study, the authors investigated the VTAVGluT2+ neuronal population response to aversive and rewarding events, both during unconditioned and conditioned stimulation. Conditioning consisted in pairing a tone (CS+) with an aversive (footshock) or rewarding (sucrose) unconditioned stimulus (US). In addition, a retrieval test was performed 24 h after conditioning to see whether VTAVGluT2+ neurons maintain a robust memory of aversive or rewarding conditioning. Finally, to better understand VTAVGluT2+ population responses to aversion and reward, we investigated their connectivity pattern using cell specific monosynaptic retrograde rabies virus tracing, allowing the mapping VTAVGluT2+ inputs.
BrainVTA offers viral vector construction & virus packaging services for AAV, LV, RABV, PRV, HSV and VSV that help researchers explore questions about genes, neurons, circuitry structure, function of brain network, mechanism and treatment of diseases.
If you have any needs, just email us at
[email protected].
