A modified rabies virus system was used for retrograde tracing to investigate the monosynaptic inputs from the CeA to Sal-Ens or Mor-Ens. AAV-DA4.4 was used to determine dopamine (DA) release. Custom-Made AAV was used for analysis of off-target effects of CRISPR-mediated genome editing. (From
BrainVTA)
The viruses used in this article from BrainVTA are in the table below
|
Custom-Made AAV |
AAV-CMV-sgRNA(Scramble)-mCherry
AAV-CMV-sgRNA(Crhr1)-mCherry
U6-sasgCrhr1-saCas9-NLS-P2A-Puro-T2A-EGFP |
|
Neurotransmitter sensors |
PT-1340 AAV-hSyn-DA4.4 |
|
AAV helper |
PT-0021 AAV-DIO-H2B-EGFP-TVA
PT-0023 AAV-DIO-RVG |
|
RV |
R01002 RV-ENVA-deltaG-dsRed |
Changyou Jiang, Xiao Yang, Guanhong He, Fan Wang, Zhilin Wang, Wendong Xu, Ying Mao, Lan Ma, Feifei Wang
Pub Date: 2021-10-12,
DOI: 10.1038/s41380-021-01321-9,
Email: [email protected]
Plasticity of neurons in the ventral tegmental area (VTA) is critical for establishment of drug dependence. However, the remodeling of the circuits mediating the transition between positive and negative effect remains unclear. Here, we used neuronal activity-dependent labeling technique to characterize and temporarily control the VTA neuronal ensembles recruited by the initial morphine exposure (morphine-positive ensembles, Mor-Ens). Mor-Ens preferentially projected to NAc, and induced dopamine-dependent positive reinforcement. Electrophysiology and rabies viral tracing revealed the preferential connections between the VTA-projective corticotrophin-releasing hormone (CRH) neurons of central amygdala (CRHCeA→VTA) and Mor-Ens, which was enhanced after escalating morphine exposure and mediated the negative effect during opiate withdrawal. Pharmacologic intervention or CRISPR-mediated repression of CRHR1 in Mor-Ens weakened the inhibitory CRHCeA→VTA inputs, and alleviated the negative effect during opiate withdrawal. These data suggest that neurons encoding opioid reward experience are inhibited by enhanced CRHCeA→VTA inputs induced by chronic morphine exposure, leading to negative effect during opiate withdrawal, and provide new insight into the pathological changes in VTA plasticity after drug abuse and mechanism of opiate dependence.
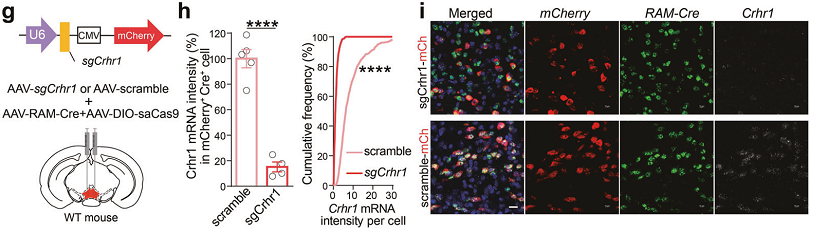 Figure 1. Pharmacological and genetic interventions of CRHR1 signaling attenuate the inhibitory inputs from CRHCeA→VTA, and the negative effect during opiate withdrawal.
Figure 1. Pharmacological and genetic interventions of CRHR1 signaling attenuate the inhibitory inputs from CRHCeA→VTA, and the negative effect during opiate withdrawal.
In this study, the authors specifically labeled neuronal ensembles recruited by initial morphine exposure in the VTA, taking the advantage of the immediate early gene-based, synaptic and neuronal activity-responsive systems. The data reveal that chronic morphine administration preferentially enhanced the GABAergic inputs of CRH neurons in the central amygdala (CeA) to VTA dopaminergic morphine ensembles, which was essential for the development of the negative effect during opiate withdrawal. The results highlight the importance of CRH neuron-mediated functional connectivity between CeA and VTA, which reduces morphine euphoria and drives the negative effect via enhanced GABAergic transmission onto the VTA ensembles encoding a drug-reward experience.
BrainVTA offers viral vector construction & virus packaging services for AAV, LV, RABV, PRV, HSV and VSV that help researchers explore questions about genes, neurons, circuitry structure, function of brain network, mechanism and treatment of diseases.
If you have any needs, just email us at
[email protected].
