A modified rabies virus system was used for retrograde tracing to confirm that posterior PF GABAergic neurons receive direct SNr PV innervation. (From
BrainVTA)
The viruses used in this article from BrainVTA are in the table below
|
Custom-Made AAV |
PT-0062 AAV-EF1a-DIO-EGFP-TVA
PT-0023 AAV-Ef1α-DIO-RVG |
|
RV |
R01002 RV-EnvA-ΔG-DsRed |
Bin Chen, Cenglin Xu, Yi Wang, Wenkai Lin, Ying Wang, Liying Chen, Heming Cheng, Lingyu Xu, Tingting Hu, Junli Zhao, Ping Dong, Yi Guo, Shihong Zhang, Shuang Wang, Yudong Zhou, Weiwei Hu, Shuming Duan, Zhong Chen
Pub Date: 2020-02-17,
DOI: 10.1038/s41467-020-14648-8,
Email: [email protected]
The precise circuit of the substantia nigra pars reticulata (SNr) involved in temporal lobe epilepsy (TLE) is still unclear. Here we found that optogenetic or chemogenetic activation of SNr parvalbumin+ (PV) GABAergic neurons amplifies seizure activities in kindling- and kainic acid-induced TLE models, whereas selective inhibition of these neurons alleviates seizure activities. The severity of seizures is bidirectionally regulated by optogenetic manipulation of SNr PV fibers projecting to the parafascicular nucleus (PF). Electrophysiology combined with rabies virus-assisted circuit mapping shows that SNr PV neurons directly project to and functionally inhibit posterior PF GABAergic neurons. Activity of these neurons also regulates seizure activity. Collectively, our results reveal that a long-range SNr-PF disinhibitory circuit participates in regulating seizure in TLE and inactivation of this circuit can alleviate severity of epileptic seizures. These findings provide a better understanding of pathological changes from a circuit perspective and suggest a possibility to precisely control epilepsy.
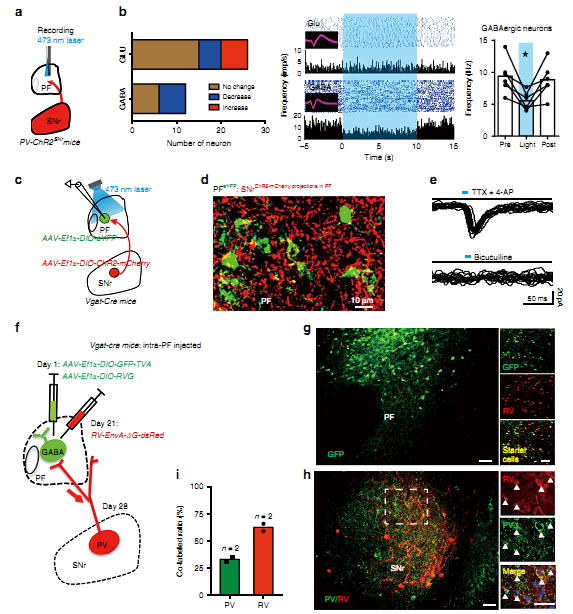 Figure 1. SNr PV neurons directly innervate PF GABAergic neurons.
Figure 1. SNr PV neurons directly innervate PF GABAergic neurons.
To study the precise circuit of the substantia nigra pars reticulata (SNr) involved in temporal lobe epilepsy (TLE),using electrophysiology combined with rabies virus-assisted circuit mapping, the authors reveal a long-range SNr-PF disinhibitory circuit that participates in regulating seizure in TLE and inactivation of this circuit can alleviate severity of epileptic seizures, which provides a better understanding of pathological changes from a circuit perspective and suggest a possibility to precisely control epilepsy.
BrainVTA offers viral vector construction & virus packaging services for AAV, LV, RABV, PRV, HSV and VSV that help researchers explore questions about genes, neurons, circuitry structure, function of brain network, mechanism and treatment of diseases.
If you have any needs, just email us at
[email protected].
