AAV-hChR2 and AAV- eNpHR3.0 were used for optogenetic manipulation. AAVs of tracing helper and RV were used for retrograde monosynaptic tracing. (From
BrainVTA)
The viruses used from BrainVTA in this article are in the table below
|
Tracing Helper |
PT-0023 rAAV-Ef1α-DIO-RVG-WPRE-pA
PT-0062 rAAV-Ef1α-DIO-EGFP-2a-TVA-WPRE-pA
PT-0095 rAAV-Ef1α-DIO-BFP-2a-TVA-WPRE-pA |
|
Optogenetic |
PT-0002 rAAV-Ef1α-DIO-hChR2 (H134R)-mCherry-WPRE-pA
PT-0001 rAAV-Ef1α-DIO-hChR2 (H134R)-eYFP-WPRE-pA
PT-0003 rAAV-Ef1α-DIO-eNpHR3.0-EYFP-WPRE-pA |
|
Control |
PT-0108 rAAV-CaMKIIa-mCherry-WPRE-pA
PT-0013 rAAV-Ef1α-DIO-mCherry-WPRE-pA
PT-0012 rAAV-DIO-EYFP-WPRE-pA |
|
RV |
R01002 RV-EnvA-ΔG-dsRed |
Weiwei Yin, Lisheng Mei, Tingting Sun, Yuping Wang, Jie Li, Changmao Chen, Zahra Farzinpour, Yu Mao, Wenjuan Tao, Juan Li, Wen Xie, Zhi Zhang
Pub Date: 2020-05-01,
DOI: 10.1097/aln.0000000000003133,
Email: [email protected]
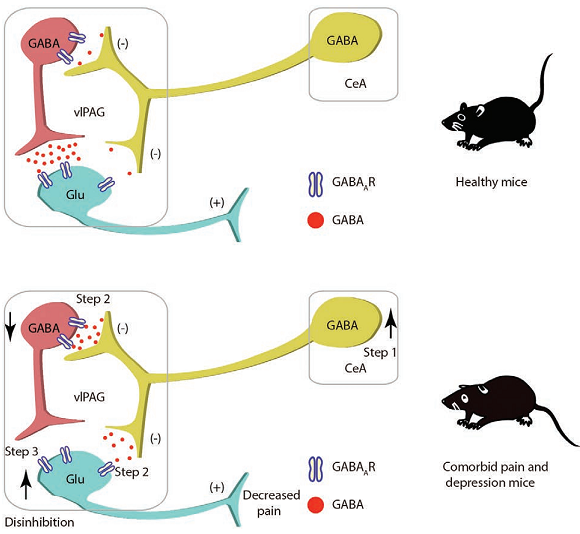
Background: The mechanisms underlying depression-associated pain remain poorly understood. Using a mouse model of depression, the authors hypothesized that the central amygdala–periaqueductal gray circuitry is involved in pathologic nociception associated with depressive states.
Methods: The authors used chronic restraint stress to create a mouse model of nociception with depressive-like behaviors. They then used retro-grade tracing strategies to dissect the pathway from the central nucleus of the amygdala to the ventrolateral periaqueductal gray. The authors performed optogenetic and chemogenetic experiments to manipulate the activity of this pathway to explore its roles for nociception.
Results: The authors found that γ-aminobutyric acid–mediated (GABAergic) neurons from the central amygdala project onto GABAergic neurons of the ventrolateral periaqueductal gray, which, in turn, locally innervate their adjacent glutamatergic neurons. After chronic restraint stress, male mice displayed reliable nociception (control, mean ± SD: 0.34 ± 0.11 g, n = 7 mice; chronic restraint stress, 0.18 ± 0.11g, n = 9 mice, P = 0.011). Comparable nociception phenotypes were observed in female mice. After chronic restraint stress, increased circuit activity was generated by disinhibition of glutamatergic neurons of the ventrolateral periaqueductal gray by local GABAergic interneurons via receiving enhanced central amygdala GABAergic inputs. Inhibition of this circuit increased nociception in chronic restraint stress mice (median [25th, 75th percentiles]: 0.16 [0.16, 0.16] g to 0.07 [0.04, 0.16] g, n = 7 mice per group, P < 0.001). In contrast, activation of this pathway reduced nociception (mean ± SD: 0.16 ± 0.08 g to 0.34 ± 0.13 g, n = 7 mice per group, P < 0.001).
Conclusions: These findings indicate that the central amygdala–ventrolateral periaqueductal gray pathway may mediate some aspects of pain symptoms under depression conditions.
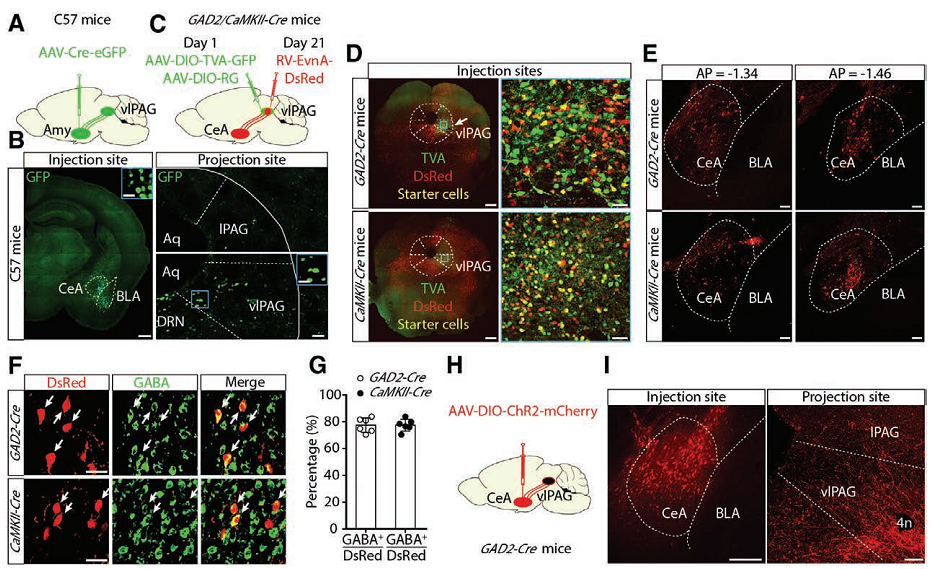 Figure 1. The central amygdala (Amy) γ-aminobutyric acid–mediated (GABAergic) neurons projects onto ventrolateral periaqueductal gray neurons.
Figure 1. The central amygdala (Amy) γ-aminobutyric acid–mediated (GABAergic) neurons projects onto ventrolateral periaqueductal gray neurons.
This study is aimed to explore the underlying mechanisms of pain symptoms in depression. The authors hypothesized that the central amygdala–periaqueductal gray circuitry is involved in the pathologic causes of pain in depression states. Using a mouse model of depression and combining chemogenetic and optogenetic experiments, the results provide a framework for how the central amygdala–periaqueductal gray circuitry may play a role in coping with nociception in depressive states.
BrainVTA offers viral vector construction & virus packaging services for AAV, LV, RABV, PRV, HSV and VSV that help researchers explore questions about genes, neurons, circuitry structure, function of brain network, mechanism and treatment of diseases.
If you have any needs, just email us at
[email protected].