AAV-hChR2 was used for optogenetic manipulation. (From
BrainVTA)
The viruses used from BrainVTA in this article are in the table below
|
Optogenetic |
PT-0992 pAAV9-CAG-hChR2(H134R)-mCherry |
|
Control |
PT-0105 pAAV9-CAG-mCherry |
Yue Cheng, Haitao Li, Long Wang, Jianyi Li, Wen Kang, Panpan Rao, Fang Zhou, Xi Wang, Congxin Huang
Pub Date: 2020-04-20,
DOI: 10.1002/jbio.202000003,
Email: [email protected]
Cardiac optogenetics facilitates the painless manipulation of the heart with optical energy and was recently shown to terminate ventricular tachycardia (VT) in explanted mice heart. This study aimed to evaluate the optogenetic‐based termination of induced VT under ischemia in an open‐chest rat model and to develop an optimal, optical‐manipulation procedure. VT was induced by burst stimulation after ligation of the left anterior descending coronary artery, and the termination effects of the optical manipulation, including electrical anti‐tachycardia pacing (ATP) and spontaneous recovery, were tested. Among different mul-tisegment optical modes, four repeated illuminations of 1000 ms in duration with 1‐second interval at a 20‐times intensity threshold on the right ventricle achieved the highest termination rate of 86.14% ± 4.145%, higher than that achieved by ATP and spontaneous termination. We demonstrated that optogenetic‐based cardioversion is feasible and effective in vivo, with the underlying mechanism involving the light‐triggered, ChR2‐induced depolarization of the illuminated myocardium, in turn generating an excitation that disrupts the preexisting re-entrant wave front.
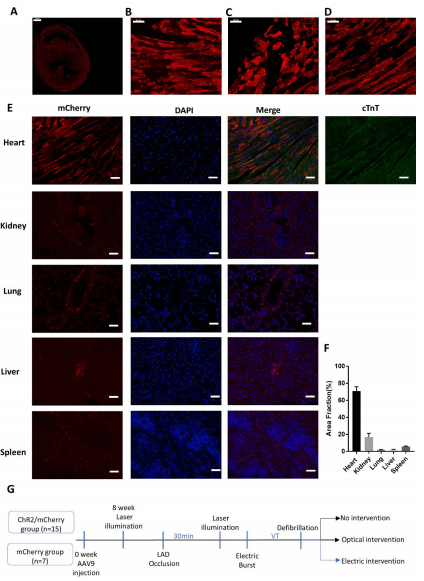 Figure 1. Internal organ transduction following high-dose systemic AAV-9 injection in SD rats (N = 6) for 10 weeks.
Figure 1. Internal organ transduction following high-dose systemic AAV-9 injection in SD rats (N = 6) for 10 weeks.
This study is aimed to evaluate the optogenetics-mediated termination of ischemia-induced VT/VF in an open-chest rat model in vivo. The authors used the light-gated cation channel channelrhodopsin-2 mutation ChR2(H134R) as the actuator and expressed it in the intact heart by adeno-associated virus serotype 9 (AAV9)-mediated gene delivery. They then illuminated the epicardium using blue laser of 473 nm as VT/VF were induced. They compared the results among different illumination protocols and the electrical ATP protocol and observed the mechanisms underlying optogenetics defibrillation.
BrainVTA offers viral vector construction & virus packaging services for AAV, LV, RABV, PRV, HSV and VSV that help researchers explore questions about genes, neurons, circuitry structure, function of brain network, mechanism and treatment of diseases.
If you have any needs, just email us at
[email protected].
