Adeno-associated virus (AAV) helpers were used for rabies virus (RV)-mediated monosynaptic tracing. (From
BrainVTA)
The viruses used in this article from BrainVTA are in the table below
|
Tracing Helper |
PT-0062 AAV-EF1a-DIO-EGFP-TVA
PT-0023 AAV-EF1a-DIO-RG |
|
RV |
R01002 EnvA-SAD1G-DsRed |
Pei Sun, Junjun Wang, Meng Zhang, Xinxin Duan, Yunfei Wei, Fuqiang Xu, Yan Ma, Yu-Hui Zhang
Pub Date: 2020-09-23,
DOI: 10.3389/fncir.2020.00053,
Email: [email protected]
As the most important organ in our bodies, the brain plays a critical role in deciding sex-related differential features; however, the underlying neural circuitry basis remains unclear. Here, we used a cell-type-specific rabies virus-mediated monosynaptic tracing system to generate a sex differences-related whole-brain input atlas of locus coeruleus noradrenaline (LC-NE) neurons. We developed custom pipelines for brain-wide comparisons of input sources in both sexes with the registration of the whole-brain data set to the Allen Mouse Brain Reference Atlas. Among 257 distinct anatomical regions, we demonstrated the differential proportions of inputs to LC-NE neurons in male and female mice at different levels. Locus coeruleus noradrenaline neurons of two sexes showed general similarity in the input patterns, but with differentiated input proportions quantitatively from major brain regions and diverse sub-regions. For instance, inputs to male LC-NE neurons were found mainly in the cerebrum, interbrain, and cerebellum, whereas inputs to female LC-NE neurons were found in the midbrain and hindbrain. We further found that specific subsets of nuclei nested within sub-regions contributed to overall sex-related differences in the input circuitry. Furthermore, among the totaled 123 anatomical regions with proportion of inputs >0.1%, we also identified 11 sub-regions with significant statistical differences of total inputs between male and female mice, and seven of them also showed such differences in ipsilateral hemispheres. Our study not only provides a structural basis to facilitate our understanding of sex differences at a circuitry level but also provides clues for future sexually differentiated functional studies related to LC-NE neurons.
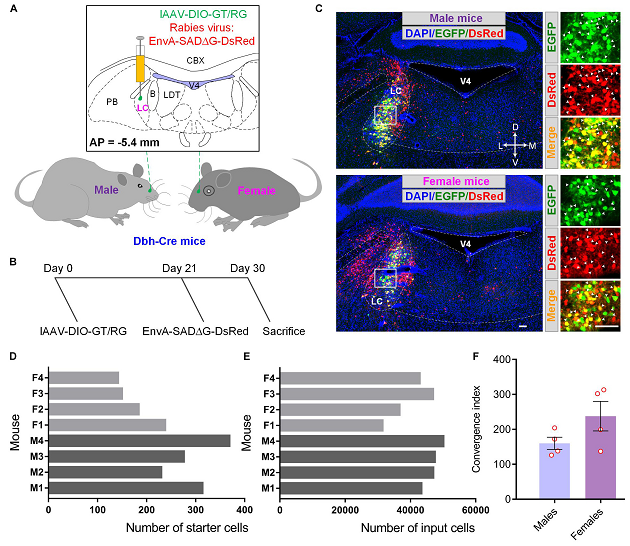 Figure 1. Demonstration of monosynaptic tracing systems to reveal the sex-related differences in LC.
Figure 1. Demonstration of monosynaptic tracing systems to reveal the sex-related differences in LC.
This study is aimed to explore the underlying neural circuitry basis of the brain in deciding sex-related differential features. Herein, the authors used cell-type-specific rabies virus-mediated monosynaptic tracing systems to map the brain-wide input sources of LC-NE neurons in male and female mice. The study thus provided a structural basis for the further sex-involved functional studies targeting LC-NE neurons.
BrainVTA offers viral vector construction & virus packaging services for AAV, LV, RABV, PRV, HSV and VSV that help researchers explore questions about genes, neurons, circuitry structure, function of brain network, mechanism and treatment of diseases.
If you have any needs, just email us at
[email protected].
