AAV-hM4D(Gi) was used for chemogenetics manipulation. (From
BrainVTA)
The viruses used in this article from BrainVTA are in the table below
|
Chemogenetics |
PT-0017 AAV-CaMKIIα-hM4D (Gi)-mCherry -WPRE-hGH polyA
PT-0488 AAV-VGAT-hM4D (Gi)-mCherry -WPRE-hGH polyA |
Yuhui Lin, Mengcheng Yao, Haiyin Wu, Feng Wu, Shiying Cao, Huanyu Ni, Jian Dong, Di Yang, Yanyu Sun, Xiaolin Kou, Jun Li, Hui Xiao, Lei Chang, Jin Wu, Yan Liu, Chunxia Luo, Dongya Zhu
Pub Date: 2021-01-27,
DOI: 10.7150/thno.53316,
Email: [email protected]
Rationale: Stroke is a leading cause of adult disability worldwide, but no drug provides functional recovery during the repair phase. Accumulating evidence demonstrates that environmental enrichment (EE) promotes stroke recovery by enhancing network excitability. However, the complexities of utilizing EE in a clinical setting limit its translation.
Methods: We used multifaceted approaches combining electrophysiology, chemogenetics, optogenetics, and floxed mice in a mouse photothrombotic stroke model to reveal the key target of EE-mediated stroke recovery.
Results: EE reduced tonic gamma-aminobutyric acid (GABA) inhibition and facilitated phasic GABA inhibition in the peri-infarct cortex, thereby promoting network excitability and stroke recovery. These beneficial effects depended on GAT-1, a GABA transporter regulating both tonic and phasic GABA signaling, as EE positively regulated GAT-1 expression, trafficking, and function. Furthermore, GAT-1 was necessary for EE-induced network plasticity, including structural neuroplasticity, input synaptic strengthening in the peri-infarct cortex, output synaptic strengthening in the corticospinal tract, and sprouting of uninjured corticospinal axons across the midline into the territory of denervated spinal cord, and functional recovery from stroke. Moreover, restoration of GAT-1 function in the peri-infarct cortex by its overexpression showed similar beneficial effects on stroke recovery as EE exposure.
Conclusion: GAT-1 is a key molecular substrate of the effects of EE on network excitability and consequent stroke recovery and can serve as a novel therapeutic target for stroke treatment during the repair phase.
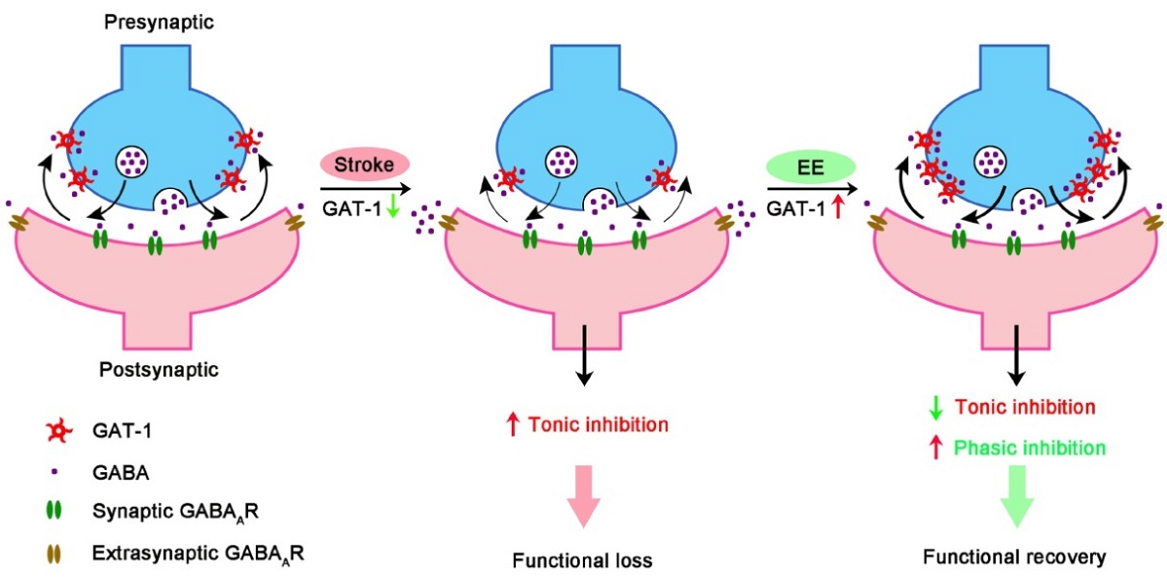 Figure 1. GAT-1 dysfunction in the peri-infarct cortex enhances tonic inhibition, thereby impairing stroke recovery. EE reduces tonic inhibition while facilitating phasic inhibition by restoring GAT-1 function and consequently promotes stroke recovery.
Figure 1. GAT-1 dysfunction in the peri-infarct cortex enhances tonic inhibition, thereby impairing stroke recovery. EE reduces tonic inhibition while facilitating phasic inhibition by restoring GAT-1 function and consequently promotes stroke recovery.
The authors used multifaceted approaches combining electrophysiology, chemogenetics, optogenetics, and floxed mice in a mouse photothrombotic stroke model to reveal the key target of EE-mediated stroke recovery. The results demonstrate that GAT-1 is a key molecular substrate of the effects of EE on network excitability and consequent stroke recovery and can serve as a novel therapeutic target for stroke treatment during the repair phase.
BrainVTA offers viral vector construction & virus packaging services for AAV, LV, RABV, PRV, HSV and VSV that help researchers explore questions about genes, neurons, circuitry structure, function of brain network, mechanism and treatment of diseases.
If you have any needs, just email us at
[email protected].
