AAV-GCaMP6s was used for fiber photometry experiments. AAV-hM3Dq and AAV-hM4D(Gi) were used for chemogenetic manipulation. AAV-ChR2 was used for optogenetic manipulation. AAV-DTA was used for selective depletion of glutamatergic neurons. (All viruses were packaged by
BrainVTA)
The viruses used in this article from BrainVTA are in the table below
|
Calcium sensors |
PT-0091 rAAV-hSyn-DIO-Gcamp6s |
|
Chemogenomic |
PT-0816 AAV-EF1a-DIO-hM3Dq-eYFP
PT-0815 AAV-EF1a-DIO-hM4Di-eYFP |
|
Optogenetic |
PT-0001 AAV-EF1a-DIO-ChR2-eYFP |
|
Control |
PT-0012 AAV-EF1a-DIO-eYFP |
|
Neuron Ablation |
PT-0775 AAV-CAG-DIO-DTA |
Chengxi Liu, Junxiao Liu, Liang Zhou, Haifeng He, Yu Zhang, Shuang Cai, Chengdong Yuan, Tianyuan Luo, Jijian Zheng, Tian Yu, Mazhong Zhang
Pub Date: 2021-03-04,
DOI: 10.3389/fnmol.2021.628996,
Email: [email protected]
Since their introduction in the 1840s, one of the largest mysteries of modern anesthesia are how general anesthetics create the state of reversible loss of consciousness. Increasing researchers have shown that neural pathways that regulate endogenous sleep–wake systems are also involved in general anesthesia. Recently, the Lateral Habenula (LHb) was considered as a hot spot for both natural sleep–wake and propofol-induced sedation; however, the role of the LHb and related pathways in the isoflurane-induced unconsciousness has yet to be identified. Here, using real-time calcium fiber photometry recordings in vivo, we found that isoflurane reversibly increased the activity of LHb glutamatergic neurons. Then, we selectively ablated LHb glutamatergic neurons in Vglut2-cre mice, which caused a longer induction time and less recovery time along with a decrease in delta-band power in mice under isoflurane anesthesia. Furthermore, using a chemogenetic approach to specifically activate LHb glutamatergic neurons shortened the induction time and prolonged the recovery time in mice under isoflurane anesthesia with an increase in delta-band power. In contrast, chemogenetic inhibition of LHb glutamatergic neurons was very similar to the effects of selective lesions of LHb glutamatergic neurons. Finally, optogenetic activation of LHb glutamatergic neurons or the synaptic terminals of LHb glutamatergic neurons in the rostromedial tegmental nucleus (RMTg) produced a hypnosis-promoting effect in isoflurane anesthesia with an increase in slow wave activity. Our results suggest that LHb glutamatergic neurons and pathway are vital in modulating isoflurane anesthesia.
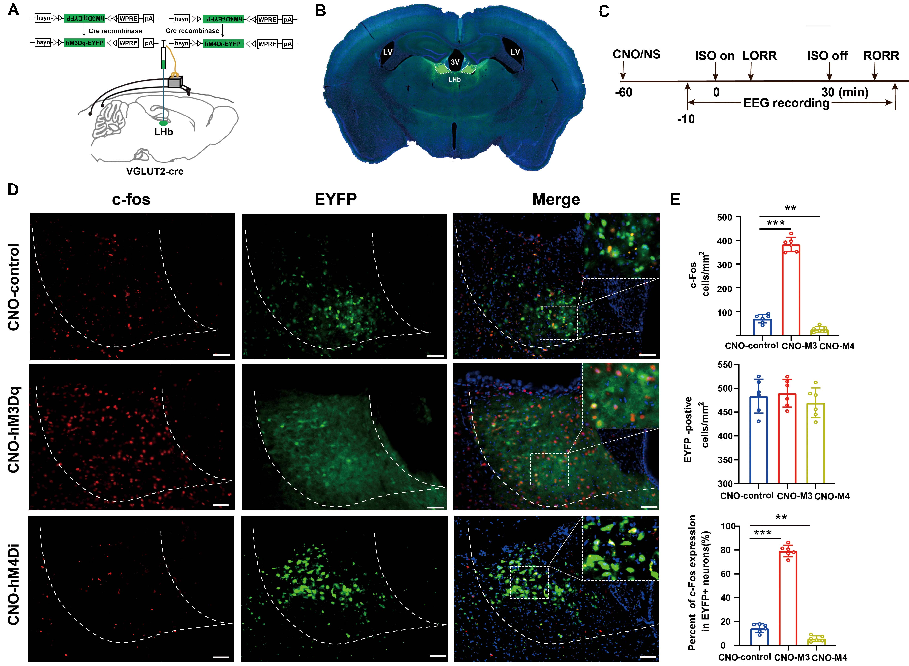 Figure 1. Activation/inactivation of LHb glutamatergic neurons induced LHb c-Fos expression during isoflurane anesthesia.
Figure 1. Activation/inactivation of LHb glutamatergic neurons induced LHb c-Fos expression during isoflurane anesthesia.
The precise mechanisms by which the general anesthetics cause the sudden reversible loss of consciousness, remain to be pinpointed. Combining calcium fiber photometry recordings, chemogenetic approach and optogenetic approach, the authors characterized and investigated how LHb glutamatergic neurons regulate isoflurane anesthesia. The findings show that LHb glutamatergic neurons play a critical role in modulating isoflurane anesthesia.
BrainVTA offers viral vector construction & virus packaging services for AAV, LV, RABV, PRV, HSV and VSV that help researchers explore questions about genes, neurons, circuitry structure, function of brain network, mechanism and treatment of diseases.
If you have any needs, just email us at
[email protected].
