Rabies virus-mediated, trans-synaptic, retrograde tracing system was used to identify the monosynaptic afferent inputs to SCN CCK neurons. (From
BrainVTA)
The viruses used in this article from BrainVTA are in the table below
|
Tracing Helper |
AAV-CAG-DIO-TVA-GFP
PT-0338 AAV-CAG-DIO-RG |
|
RV |
R01002 RV-EvnA-DG-dsRed |
Xiang-Shan Yuan, Hao-Hua Wei, Wei Xu, Lu Wang, Wei-Min Qu, Rui-Xi Li and Zhi-Li Huang
Pub Date: 2018-11-05,
DOI: 10.3389/fnins.2018.00807,
Email: sales@brainvta.com
The suprachiasmatic nucleus (SCN) is the principal pacemaker driving the circadian rhythms of physiological behaviors. The SCN consists of distinct neurons expressing neuropeptides, including arginine vasopressin (AVP), vasoactive intestinal polypeptide (VIP), gastrin-releasing peptide (GRP), cholecystokinin (CCK), and so on. AVP, VIP, and GRP neurons receive light stimulation from the retina to synchronize endogenous circadian clocks with the solar day, whereas CCK neurons are not directly innervated by retinal ganglion cells and may be involved in the non-photic regulation of the circadian clock. To better understand the function of CCK neurons in non-photic circadian rhythm, it is vital to clarify the direct afferent inputs to CCK neurons in the SCN. Here, we utilized a recently developed rabies virus- and Cre/loxP-based, cell type-specific, retrograde tracing system to map and quantitatively analyze the whole-brain monosynaptic inputs to SCN CCK neurons. We found that SCN CCK neurons received direct inputs from 29 brain nuclei. Among these nuclei, paraventricular nucleus of the hypothalamus (PVH), paraventricular nucleus of the thalamus (PVT), supraoptic nucleus (SON), ventromedial nucleus of the hypothalamus, and seven other nuclei sent numerous inputs to CCK neurons. Moderate inputs originated from the zona incerta, periventricular hypothalamic nucleus, and five other nuclei. A few inputs to CCK neurons originated from the orbital frontal cortex, prelimbic cortex, cingulate cortex, claustrum, and seven other nuclei. In addition, SCN CCK neurons were preferentially innervated by AVP neurons of the ipsilateral PVH and SON rather than their contralateral counterpart, whereas the contralateral PVT sentmore projections to CCK neurons than to its ipsilateral counterpart. Taken together, these results expand our knowledge of the specific innervation to mouse SCN CCK neurons and provide an important indication for further investigations on the function of CCK neurons.
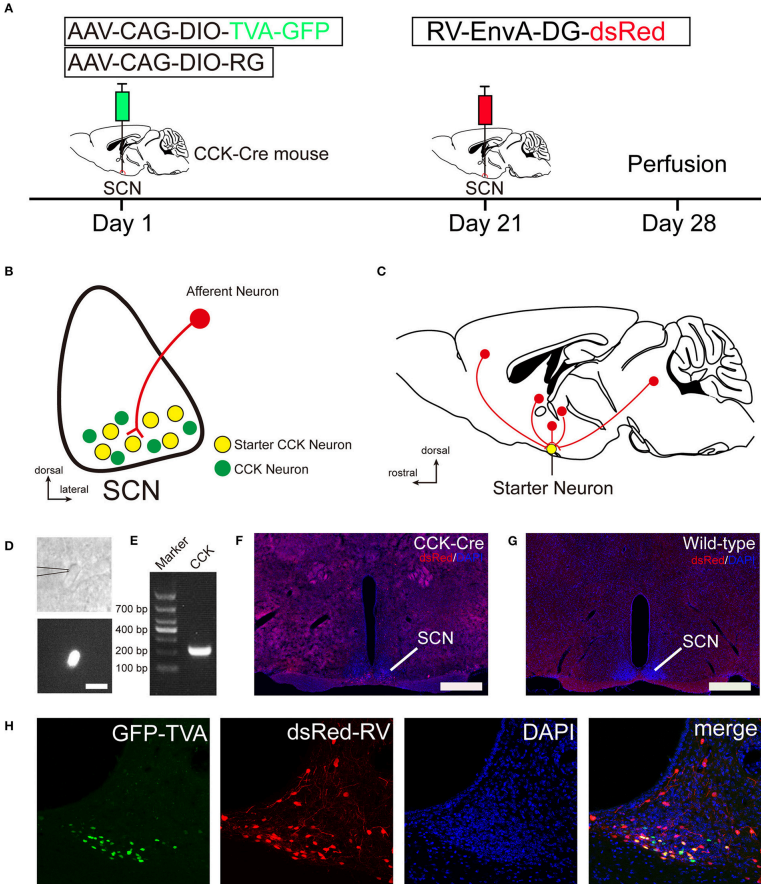
Figure. 1 Monosynaptic afferent tracing on SCN CCK neurons with a rabies virus-based, retrograde tracing system.
In this study, the authors utilized this viral tracing system to map the whole-brain afferent inputs to SCN CCK neurons. They found 29 afferent brain nuclei, including several important nuclei that integrated circadian, ingestive, and osmotic information to SCN CCK neurons. The quantitative results provide numerous evidence for the structural framework of SCN CCK neurons and can guide further investigations of neuronal pathways thatmediate functions of CCK neurons in the SCN.
BrainVTA offers viral vector construction & virus packaging services for AAV, LV, RABV, PRV, HSV and VSV that help researchers explore questions about genes, neurons, circuitry structure, function of brain network, mechanism and treatment of diseases.
If you have any needs, just email us at
sales@brainvta.com.
