Drd1-mCherry and Drd2-mCherry virus (From
BrainVTA) were used to specifically label the D1-MSNs or D2-MSNs to observe the effects of Rac1 on D1-MSNs and D2-MSNs spine remodelling in the NAc.
The viruses used in this article from BrainVTA are in the table below
|
Control |
AAV-Drd1-mCherry
PT-0367 AAV-Drd2-mCherry |
Pub Date: 2019-09-21,
DOI: 10.7150/thno.34655,
Email: sales@brainvta.com
Jinlan Zhao, Li Ying, Yutong Liu, N Liu, Genghong Tu, Mengjuan Zhu, Yue Wu, Bin Xiao, Liuzhen Ye, Juan Li, Fukun Guo, Lin Zhang, Huijun Wang and Lu Zhang
Rationale: Repeated methamphetamine (METH) exposure induces long-term cognitive deficits and pathological drug-associated memory that can be disrupted by manipulating memory reconsolidation and extinction. The nucleus accumbens (NAc) is the key region of the brain reward system and predominantly consists of two subtypes of medium spiny neurons (MSNs) based on the expression of D1 or D2 dopamine receptors (D1-MSNs or D2-MSNs). Spine structural plasticity in the NAc is critical for the acquisition, reconsolidation and extinction of drug-associated memory. However, the molecular mechanisms underlying METH-associated memory and spine remodelling in each type of MSNs in the NAc remain unknown. Here, we explored whether Rac1 in the NAc mediates METH-associated contextual memory and spine remodelling.
Methods: Pharmacological and genetic manipulations of Rac1 were used to investigate its role during the acquisition, reconsolidation and extinction of METH-associated contextual memory. Recombinant adeno-associated viruses expressing mCherry under the control of the dopamine D1 receptor gene promoter (Drd1-mCherry) or dopamine D2 receptor gene promoter (Drd2-mCherry) were used to specifically label D1-MSNs or D2-MSNs.
Results: Using viral-mediated gene transfer, we demonstrated that decreased Rac1 activity was required for the acquisition of METH-associated contextual memory and the METH-induced increase in thin spine density, whereas increased Rac1 signalling was important for the extinction of METH-associated contextual memory and the related elimination of thin spines. Moreover, the increase of dendritic spines was both found in D1-MSNs and D2-MSNs during the acquisition process, but extinction training selectively decreased the spine density in D1-MSNs. Interestingly, Rac1 was responsible for METH-induced spine plasticity in D1-MSNs but not in D2-MSNs. Additionally, we found that microinjection of a Rac1 inhibitor or activator into the NAc was not sufficient to disrupt reconsolidation, and the pharmacological activation of Rac1 in the NAc facilitated the extinction of METH-associated contextual memory. Regarding cognitive memory, decreased Rac1 activity improved the METH-induced impairment in object recognition memory.
Conclusion: Our findings indicate that Rac1 plays opposing roles in the acquisition and extinction of METH-associated contextual memory and reveal the cell-specific role of Rac1 in METH-associated spine remodelling, suggesting that Rac1 is a potential therapeutic target for reducing relapse in METH addiction and remediating METH-induced recognition memory impairment.
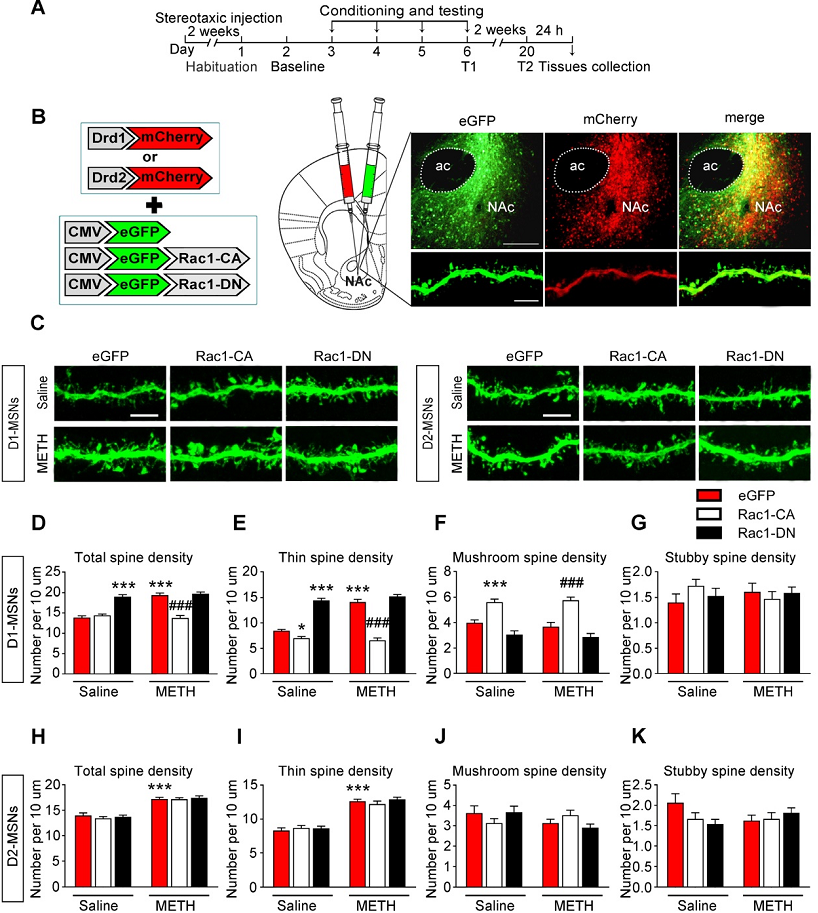 Fig1. Decreased Rac1 signalling mediates spine remodelling in D1-MSNs during the acquisition of METH-associated contextual memory
Fig1. Decreased Rac1 signalling mediates spine remodelling in D1-MSNs during the acquisition of METH-associated contextual memory
The researchers explored whether Rac1 in the NAc mediates METH-associated contextual memory and spine remodeling in this study, using viral-mediated gene transfer and pharmacological manipulation, the results show that Rac1 plays opposing roles in the acquisition and extinction of METH-associated contextual memory and reveal the cell-specific role of Rac1 in METH-associated spine remodelling, suggesting that Rac1 is a potential therapeutic target for reducing relapse in METH addiction and remediating METH-induced recognition memory impairment.
BrainVTA offers viral vector construction & virus packaging services for AAV, LV, RABV, PRV, HSV and VSV that help researchers explore questions about genes, neurons, circuitry structure, function of brain network, mechanism and treatment of diseases.
If you have any needs, just email us at
sales@brainvta.com.
