AAV expressing the Cre enzyme
(From BrainVTA) was used to induce NAc-specific deletion of D1Rs (NAc Drd1KO) and D2Rs (NAc Drd2KO). Lentiviruses were used to evaluate the effects of Rac1 and Cdc42 on METH-induced behavioral and structural plasticity.
The viruses used in this article from BrainVTA are in the table below
|
Contrrol |
PT-0100 rAAV-hSyn-mCherry-WPRE-hGH polyA |
|
CRE Recombinase |
PT-0407 rAAV-hSyn-CRE-mCherry-WPRE-hGH polyA |
Genghong Tu, Li Ying, Liuzhen Ye, Jinlan Zhao, Nuyun Liu, Juan Li, Yutong Liu, Mengjuan Zhu, Yue Wu, Bin Xiao, Huidong Guo, Fukun Guo, Huijun Wang, Lin Zhang, and Lu Zhang
Pub Date: 2019-05-15,
DOI: 10.1016/j.biopsych.2019.03.966,
Email: [email protected]
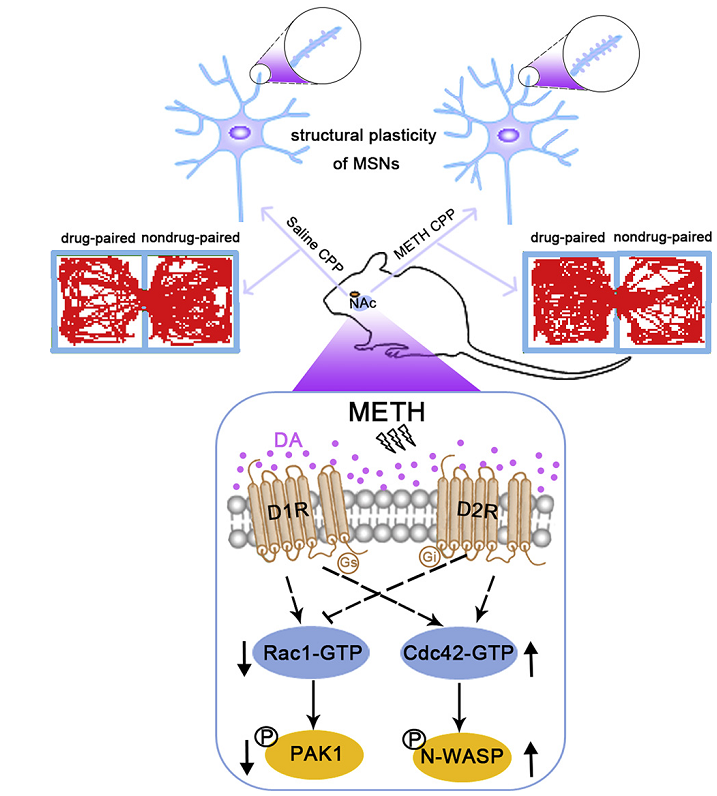
BACKGROUND: Methamphetamine (METH) is a highly addictive psychostimulant that strongly activates dopamine receptor signaling in the nucleus accumbens (NAc). However, how dopamine D1 and D2 receptors (D1Rs and D2Rs, respectively) as well as downstream signaling pathways, such as those involving Rac1 and Cdc42, modulate METH-induced behavioral and structural plasticity is largely unknown.
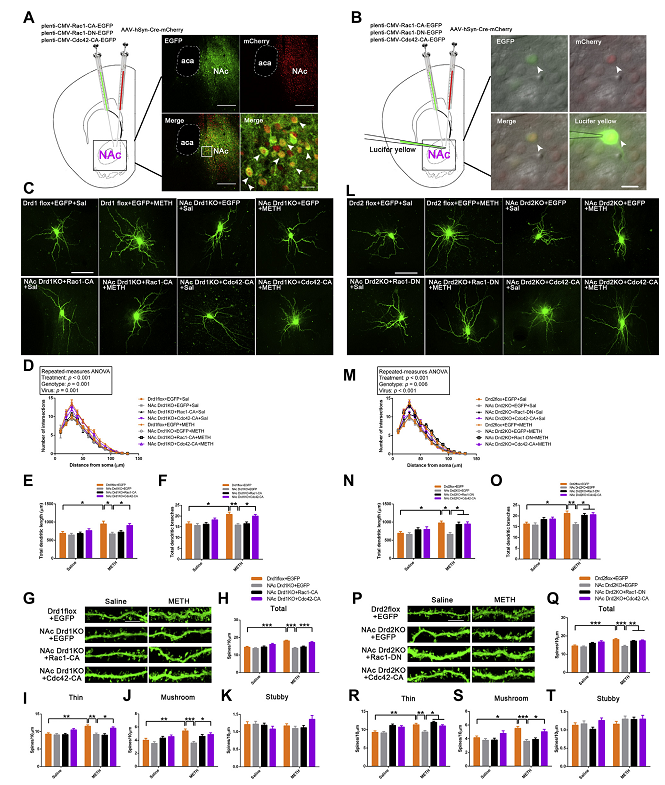
METHODS: Using NAc conditional D1R and D2R deletion mice, Rac1 and Cdc42 mutant viruses, and a series of behavioral and morphological methods, we assessed the effects of D1Rs and D2Rs on Rac1 and Cdc42 in modulating METH-induced behavioral and structural plasticity in the NAc.
RESULTS: D1Rs and D2Rs in the NAc consistently regulated METH-induced conditioned place preference, locomotor activation, and dendritic and spine remodeling of medium spiny neurons but differentially regulated METH withdrawal–induced spatial learning and memory impairment and anxiety. Interestingly, Rac1 and Cdc42 signaling were oppositely modulated by METH, and suppression of Rac1 signaling and activation of Cdc42 signaling were crucial to METH-induced conditioned place preference and structural plasticity but not to locomotor activation. D1Rs activated Rac1 and Cdc42 signaling, while D2Rs inhibited Rac1 signaling but activated Cdc42 signaling to mediate METH-induced conditioned place preference and structural plasticity but not locomotor activation. In addition, NAc D1R deletion aggravated METH withdrawal–induced spatial learning and memory impairment by suppressing Rac1 signaling but not Cdc42 signaling, while NAc D2R deletion aggravated METH withdrawal–induced anxiety without affecting Rac1 or Cdc42 signaling.
CONCLUSIONS: D1Rs and D2Rs differentially regulate Rac1 and Cdc42 signaling to modulate METH-induced behavioral plasticity and the structural remodeling of medium spiny neurons in the NAc.
Figure 1. Dopamine D1 and D2 receptors (D1Rs and D2Rs, respectively) modulate methamphetamine (METH)-induced structural plasticity by differentially regulating Rac1 and Cdc42 signaling in the nucleus accumbens (NAc).
In this study, using NAc conditional D1R and D2R deletion mice and Rac1 and Cdc42 mutant viruses, the authors found that D1Rs and D2Rs differentially regulate Rac1 and Cdc42 signaling to modulate METH-induced behavioral and structural plasticity. The findings suggest that the transmission of dopamine receptor signals to downstream molecules, such as Rac1 and/or Cdc42, may serve as a therapeutic target for the treatment of drug addiction.
BrainVTA offers viral vector construction & virus packaging services for AAV, LV, RABV, PRV, HSV and VSV that help researchers explore questions about genes, neurons, circuitry structure, function of brain network, mechanism and treatment of diseases.
If you have any needs, just email us at
[email protected].